17 Thermochemistry Vocabulary Review Answers
- Slides: 29
Download presentation

Thermochemistry Chapter 17

Thermochemistry is the report of estrus changes that occur during chemic reactions.
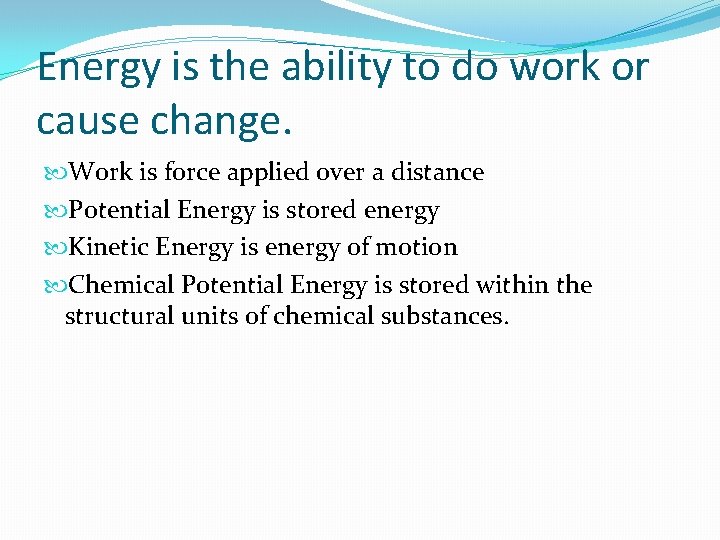
Energy is the ability to do piece of work or cause change. Piece of work is force applied over a distance Potential Energy is stored energy Kinetic Energy is energy of motion Chemical Potential Free energy is stored within the structural units of chemical substances.
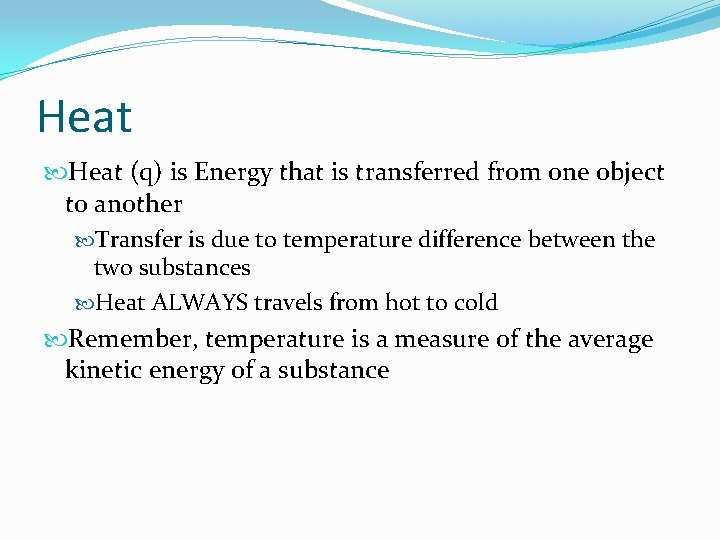
Heat (q) is Energy that is transferred from i object to another Transfer is due to temperature difference between the ii substances Heat ALWAYS travels from hot to cold Remember, temperature is a measure of the average kinetic energy of a substance

Oestrus CANNOT be detected. the changes Caused by heat nevertheless can be ex. Rise in temperature

Law of Conservation of Energy In any chemical or concrete process, energy is neither created nor destroyed. All of the energy involved can be accounted for as Piece of work, Potential Energy, or Heat.
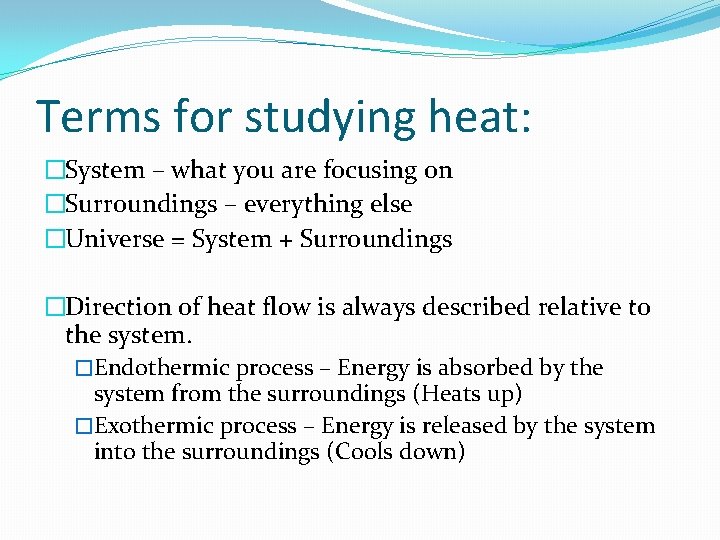
Terms for studying rut: �System – what you are focusing on �Surroundings – everything else �Universe = System + Surroundings �Management of oestrus flow is always described relative to the arrangement. �Endothermic procedure – Free energy is absorbed by the arrangement from the surroundings (Heats upward) �Exothermic procedure – Energy is released by the system into the surroundings (Cools down)
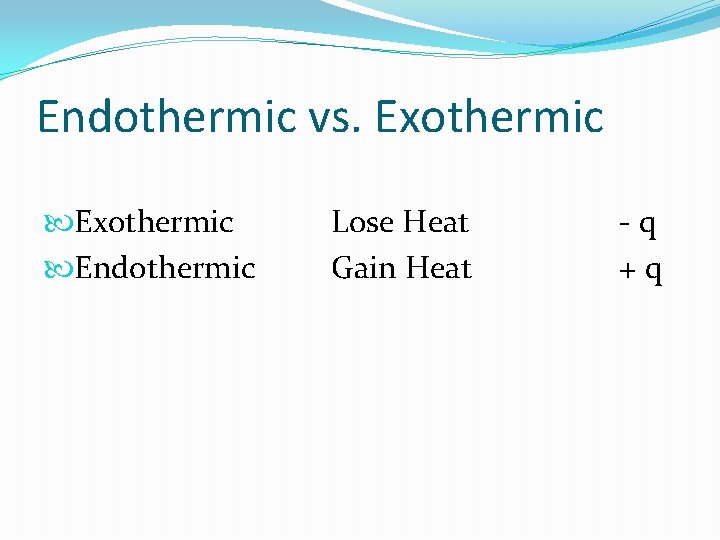
Endothermic vs. Exothermic Endothermic Lose Rut Gain Rut - q + q
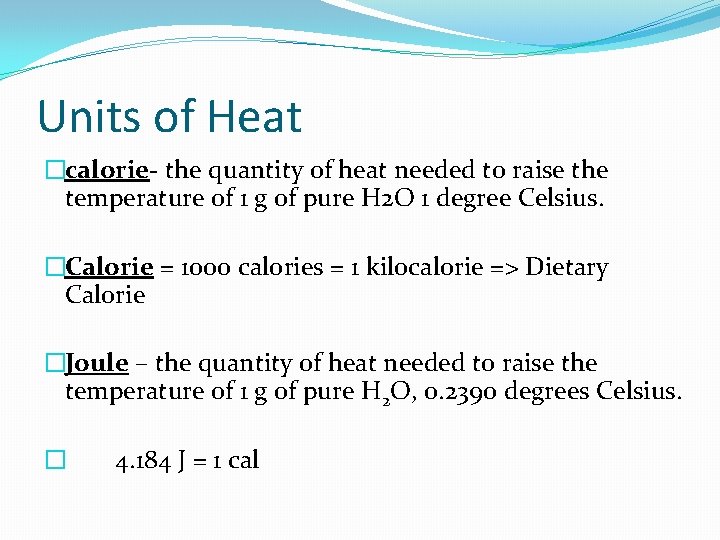
Units of Estrus �calorie- the quantity of oestrus needed to heighten the temperature of ane g of pure H 2 O i degree Celsius. �Calorie = m calories = i kilocalorie => Dietary Calorie �Joule – the quantity of heat needed to enhance the temperature of 1 thou of pure H 2 O, 0. 2390 degrees Celsius. � four. 184 J = 1 cal
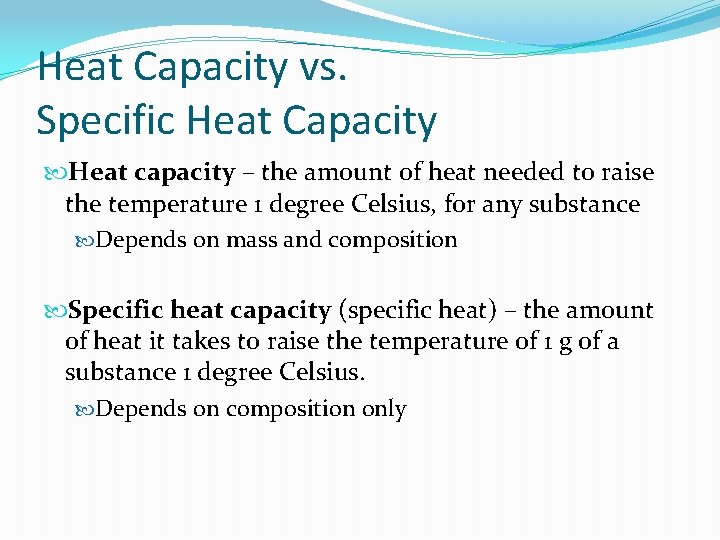
Oestrus Chapters vs. Specific Heat Capacity Heat capacity – the corporeality of estrus needed to raise the temperature ane degree Celsius, for whatever substance Depends on mass and limerick Specific heat capacity (specific estrus) – the amount of rut it takes to raise the temperature of 1 g of a substance 1 degree Celsius. Depends on limerick only
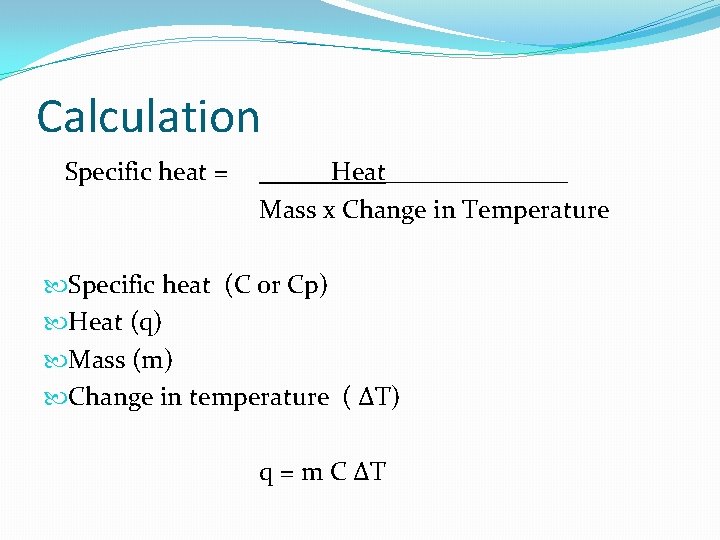
Calculation Specific estrus = Heat_______ Mass x Change in Temperature Specific rut (C or Cp) Estrus (q) Mass (chiliad) Modify in temperature ( ∆T) q = chiliad C ∆T
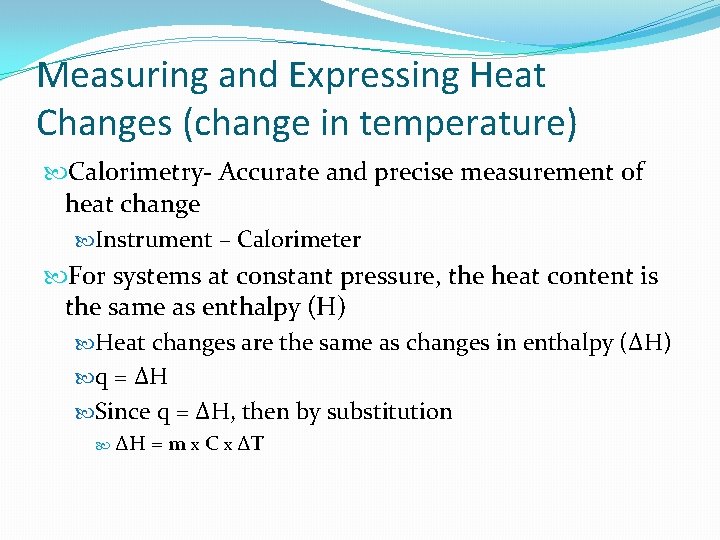
Measuring and Expressing Heat Changes (change in temperature) Calorimetry- Accurate and precise measurement of heat modify Musical instrument – Calorimeter For systems at constant pressure level, the heat content is the same as enthalpy (H) Estrus changes are the same as changes in enthalpy (ΔH) q = ΔH Since q = ΔH, then by substitution ΔH = m 10 C x ΔT
Heat in Changes of Country Molar Heat of Fusion (ΔHfus) – Solid to Liquid Molar Heat of Vaporization (ΔHvap) – Liquid to Gas q = ΔH x mass OR q = ΔH x moles Depends on units of ΔH
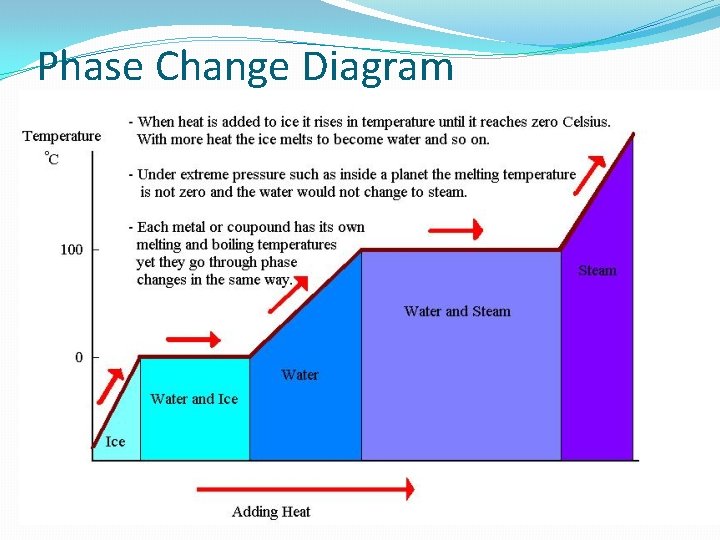
Stage Change Diagram
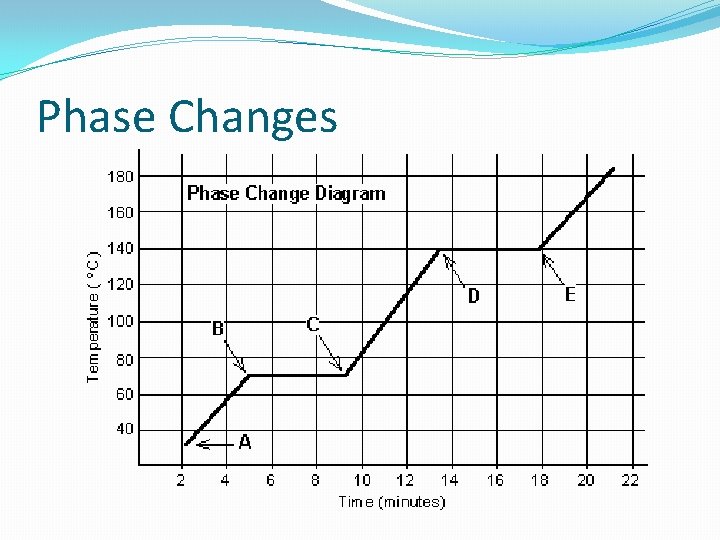
Phase Changes

Grouping Concept Questions Your text defines free energy equally "the ability to do work or to cause change". Another definition of free energy is "the ability to resist a natural tendency". Explain this definition and provide an case. A friend of yours reads that the process of water freezing is exothermic. This friend tells yous that this can't exist truthful because exothermic implies "hot", and ice is cold. Is the procedure of water freezing exothermic? If and so, explain information technology then your friend tin sympathise it. If non, explain why not.

Group Concept Questions You place hot metal into a chalice of cold water. Somewhen what is true about the temperature of the metal compared to that of the h2o? Explain why this is true. Label this process as endothermic or exothermic if we consider the system to be: the metal. Explain the water. Explicate
Grouping Concept Questions The text describes the constabulary of conservation of energy. Is at that place a law of conservation of estrus? Explicate why or why not. What does it mean when the heat for a process is reported with a negative sign? You place 100. 0 g of a hot metallic in 100. 0 g of common cold water. Which substance (metal or h2o) undergoes a larger temperature change? Why is this?

Group Concept Questions A desert is very hot during mean solar day simply quite common cold at night. In the Midwest of the United States, the temperature is more than constant between day and night in the summer. Why is this? Explain why aluminum cans make proficient storage containers for soft drinks.
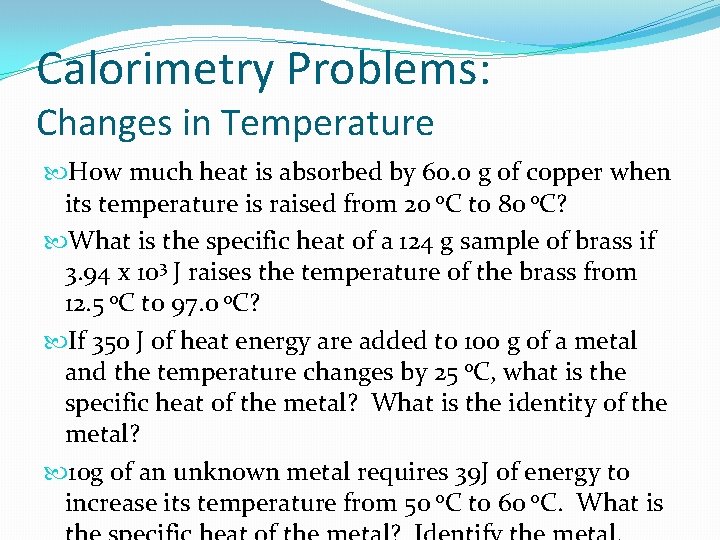
Calorimetry Problems: Changes in Temperature How much heat is captivated by 60. 0 m of copper when its temperature is raised from 20 o. C to 80 o. C? What is the specific oestrus of a 124 g sample of contumely if 3. 94 x 103 J raises the temperature of the brass from 12. 5 o. C to 97. 0 o. C? If 350 J of heat energy are added to 100 g of a metallic and the temperature changes past 25 o. C, what is the specific oestrus of the metal? What is the identity of the metal? 10 g of an unknown metallic requires 39 J of free energy to increase its temperature from 50 o. C to 60 o. C. What is
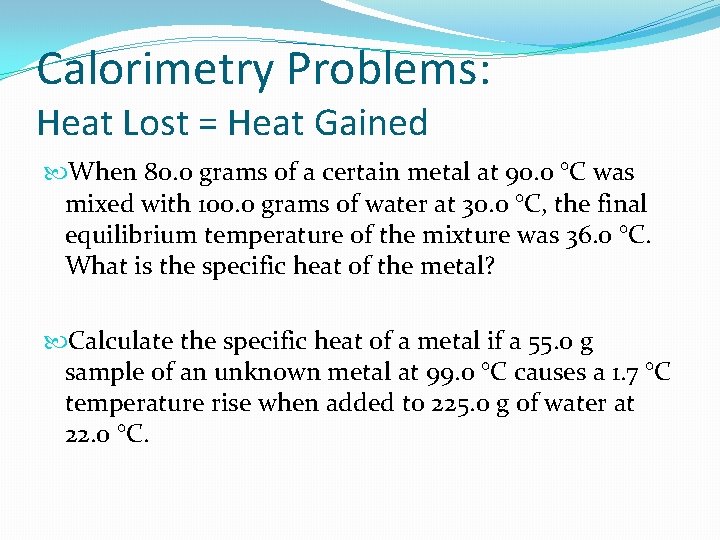
Calorimetry Bug: Heat Lost = Heat Gained When 80. 0 grams of a certain metal at 90. 0 °C was mixed with 100. 0 grams of water at xxx. 0 °C, the terminal equilibrium temperature of the mixture was 36. 0 °C. What is the specific heat of the metallic? Calculate the specific heat of a metal if a 55. 0 grand sample of an unknown metallic at 99. 0 °C causes a 1. vii °C temperature rise when added to 225. 0 g of h2o at 22. 0 °C.
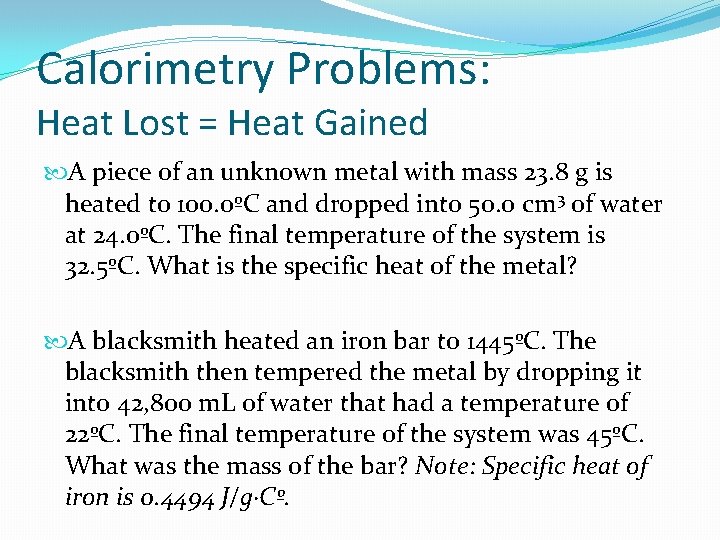
Calorimetry Bug: Heat Lost = Oestrus Gained A slice of an unknown metallic with mass 23. 8 g is heated to 100. 0ºC and dropped into l. 0 cm 3 of h2o at 24. 0ºC. The final temperature of the system is 32. 5ºC. What is the specific heat of the metal? A blacksmith heated an atomic number 26 bar to 1445ºC. The blacksmith so tempered the metallic past dropping it into 42, 800 m. L of water that had a temperature of 22ºC. The final temperature of the system was 45ºC. What was the mass of the bar? Annotation: Specific rut of fe is 0. 4494 J/yard·Cº.

Energy and Reactions How does Free energy impact REACTIONS

Enthalpy The corporeality of energy gained or released in a reaction is the ENTHALPY (∆H). ALL reactions require energy to occur. The amount of energy needed to occur is chosen the ACTIVATION Energy. The more energy required for the reaction to occur, the less likely the reaction will happen. Because of the energy released in exothermic reactions, exothermic reactions are more likely to occur than endothermic reactions. In other words, they are more spontaneous.
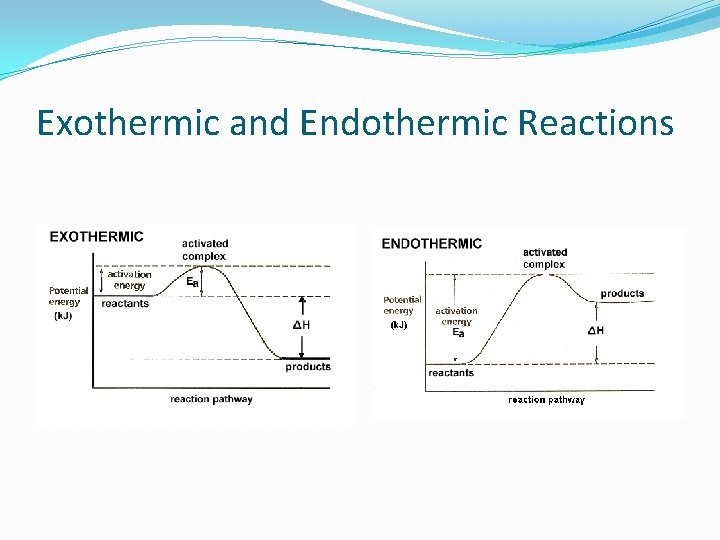
Exothermic and Endothermic Reactions
Catalysts speed upward reactions by lowering the activation energy required for the reaction to occur. Enzymes are biological catalysts. Well-nigh catalysts piece of work by helping ions and molecules to "line upwards" the right way so they tin react. http: //www. dlt. ncssm. edu/tiger/Wink/kinetics/Enzyme. Goad. html
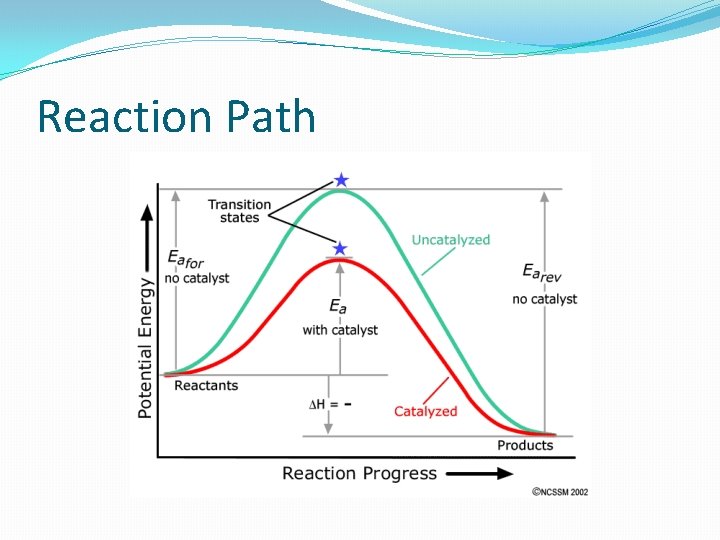
Reaction Path

Entropy Whether or non a reaction volition occur depends on both ENTHALPY and ENTROPY. Entropy is a measure of disorder. Increasing disorder is a spontaneous procedure. Entropy of States: solid < liquid < gas Kinetic. . Energy – Gas Kinetic Energy – Liquid Kinetic Free energy – Solid Mixing substances, combining or separating elements, and changing the temperature can impact the level of disorder besides. Mixing gases

Spontaneous? If energy is released and disorder is increased, the reaction will be spontaneous. In other words, it volition happen "on its own. " If energy is absorbed and disorder decreases, the reaction volition be non-spontaneous. These reactions will need "help" to occur. They can't practise it past themselves. If 1 factor is favorable and one is unfavorable, then spontaneity will depend on the values of enthalpy and entropy.
17 Thermochemistry Vocabulary Review Answers,
Source: https://slidetodoc.com/thermochemistry-chapter-17-thermochemistry-is-the-study-of/
Posted by: wallaceuple1986.blogspot.com


0 Response to "17 Thermochemistry Vocabulary Review Answers"
Post a Comment